The alternative to sodium hypochlorite
Looking for a powerful disinfectant with the same application as sodium hypochlorite, but with a much lower chance of residues such as chlorite and/or chlorate? Discover the advantages of our disinfectant over NaOCl below.
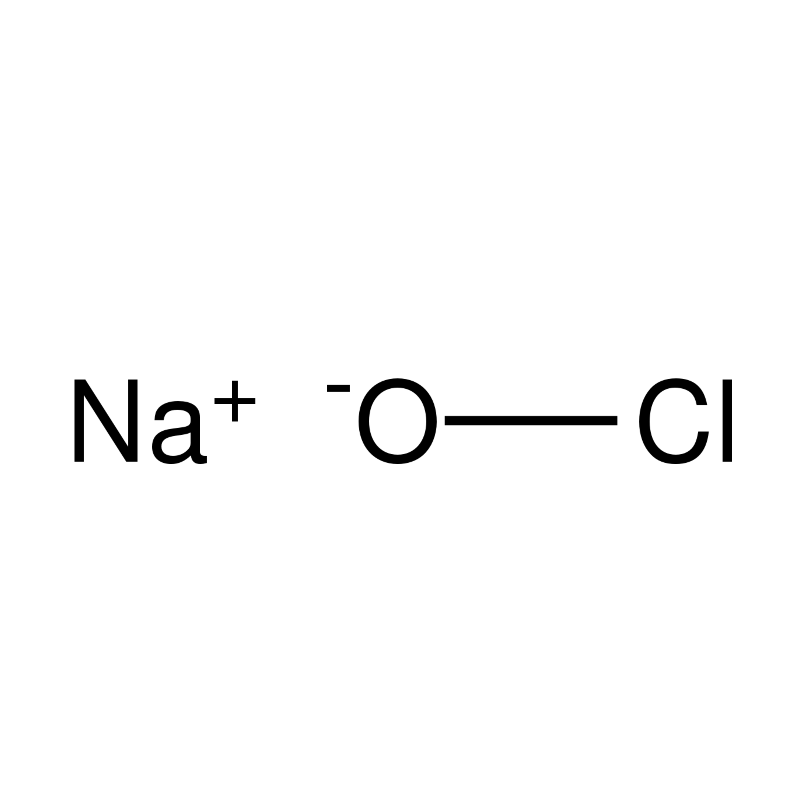
What is sodium hypochlorite (NaOCl)?
Sodium hypochlorite (NaOCl) is a well-known disinfectant widely used for water and surface disinfection. It is also the active ingredient in many common bleaching agents. sodium hypochlorite is produced via electrolysis of sodium chloride in water, or by reacting chlorine gas with sodium hydroxide [1]. This results in a strong oxidiser that can effectively combat many micro-organisms.

What is sodium hypochlorite used for?
Sodium hypochlorite is widely used in various sectors:
- Domestic and industrial cleaning: for cleaning and disinfection of surfaces and sanitary facilities.
- Medical sector: in lower concentrations, for example in dental treatment such as root canal treatments.
- Water treatment: disinfecting drinking water.
- Swimming pools: disinfecting pool water to keep it free of microbiological contamination.
- Process water in industry: in factories to disinfect their process water.
Disadvantages Sodium Hypochlorite
Despite its wide applicability, sodium hypochlorite has some significant drawbacks:
- High risk of disinfection by-products. Sodium hypochlorite can form chlorite or glorate as unwanted residues (disinfection by-products) at high doses. This risk is much higher than with alternatives such as HOCl. These disinfection by-products can impact both human health and the environment. It is risky for foodstuffs, but also for the health of your employees.
- Plastic waste. In many cases, sodium hypochlorite is purchased in barrels and/or jerry cans. These create a large waste stream within companies, contributing to the environmental burden. The storage space demanded by the purchases should not be forgotten either.
- CO2 emission. The transport and storage of large quantities of sodium hypochlorite cause increased CO2 emissions. These have to be delivered on site, which involves the necessary fuel consumption.
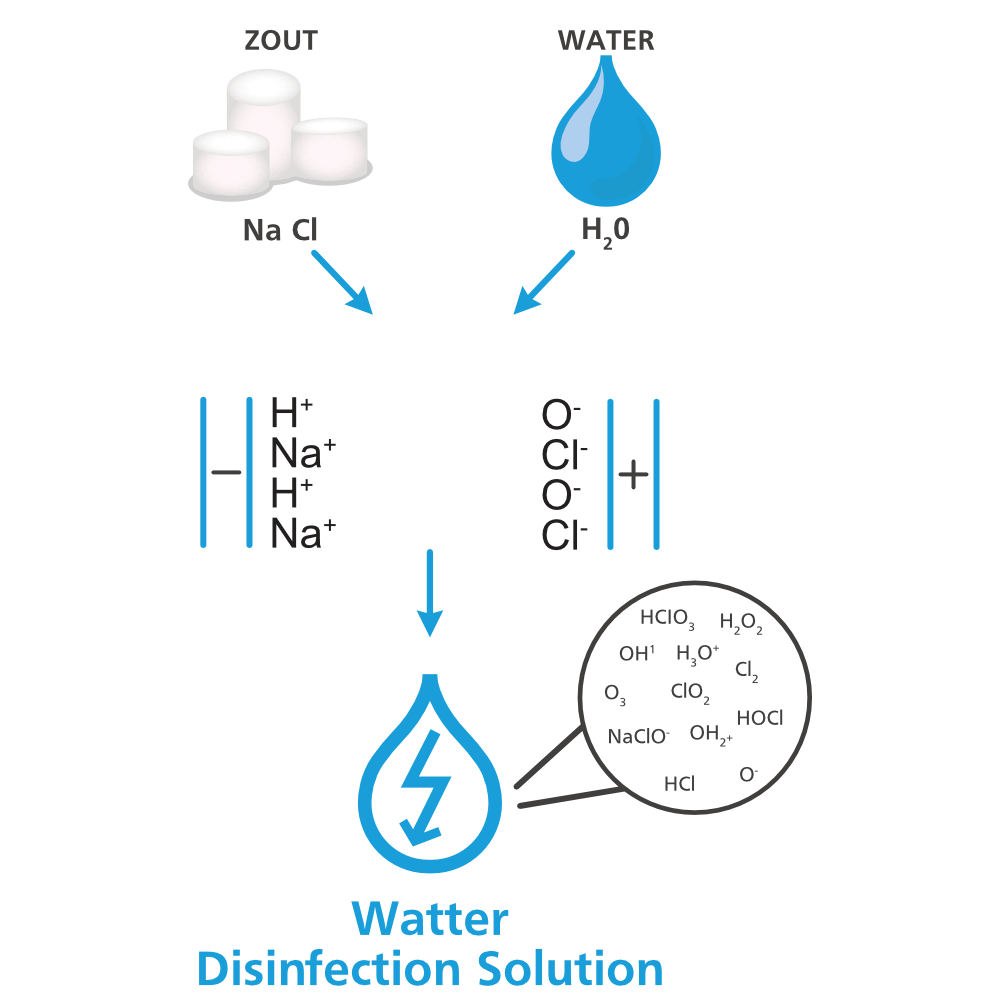
The alternative: Watter Disinfection Solution
Watter Disinfection Solution is produced sustainably on site with our Watter technology. This eliminates the need for packaging and transport. The agent is considered user-friendly in effective concentrations [2]. But what makes Watter Disinfection Solution a good alternative to sodium hypochlorite?
How does Watter Disinfection Solution work?
The active ingredient in Watter Disinfection Solution is HOCl (also known as hypochlorous acid). Due to our innovative Watter technology, we can produce it on site (in-situ) by electrolyzing salt dissolved in water. Because HOCl has an oxidising property, this disinfectant is highly effective against fungi, yeasts, bacteria and viruses. This disinfectant can be used in many areas where hygiene is very important. For example, it is widely used for drinking water disinfection in livestock farming and process water disinfection in industry.
What is the difference between hypochlorous acid and sodium hypochlorite?
|
Hypochlorous acid |
Sodium Hypochlorite |
|
|
Wetenschappelijke formule |
HOCl |
NaOCl |
|
Production |
Elektrolysis of water and salt |
Through a chemical reaction between chlorine a sodium hydroxide |
|
Kleur |
Transparent |
Pale, greenish yellow |
|
Effective |
Effective against:
|
Effective against(At a 250x higher concentration than HOCL)
|
|
Side Effects |
|
|
|
Warning Symbols |
N/A |
  |
Convinced?
That Watter Disinfection Solution is also the perfect alternative to Sodium Hypochlorite for you?
References
- Sanfourche, Gardent, Bull. Soc. Chim. [4] 35, 1089 (1924); Schmeisser in Handbook of Preparative Inorganic Chemistry vol. 1, G. Brauer, Ed. (Academic Press, New York, 2nd ed., 1963) pp 309-310
- Tsai, SY., Liu, YM., Lin, ZW. et al. Antimicrobial activity effects of electrolytically generated hypochlorous acid-treated pathogenic microorganisms by isothermal kinetic simulation. J Therm Anal Calorim 148, 1613–1627 (2023). https://doi.org/10.1007/s10973-022-11727-4



