Psst… There is a better alternative for peracetic acid!
But what is peracetic acid?
Peracetic acid is a mixture of acetic acid and hydrogen peroxide. It is a clear and colourless liquid with a characteristic acetic odour. It is produced by reacting acetic acid with hydrogen peroxide.
Peracetic acid is an agent commonly used in the food preparation and treatment industry as a disinfectant and cleaning agent. Because it is effective against bacteria and fungi, it has also been used to treat fruit and vegetables since the 50s.
Peracetic acid is an organically linked acetic acid. It is used as a disinfectant against bacteria, fungi and viruses. Peracetic acid is a very reactive substance. However, its action is also harmful to humans. Appropriate safety measures must therefore be taken when using it [1]. However, there is an equally effective alternative that does not require safety measures during use.

What is Watter Disinfection Solution?
Watter Disinfection Solution is produced sustainably on site with our Watter technology. This eliminates the need for packaging and transport. In effective concentrations, the agent is considered user-friendly [2]. But what makes Watter Disinfection Solution a good alternative to peracetic acid?
How does Watter Disinfection Solution work?
The main active ingredient in Watter Disinfection Solution is HOCl (also known as hypochlorous acid). Our innovative Watter technology allows us to produce it on site by electrolyzing salt dissolved in water. Because HOCl has an oxidising property, this disinfectant is very effective against fungi, yeasts, bacteria and viruses [3]. This disinfectant can be used in many areas where hygiene is very important. For example, it is widely used in drinking water disinfection in animal farming and process water disinfection in industry. Watter Disinfection Solution has proven its effectiveness with our customers, are you ready for innovation for your business?
|
Hypochlorous acid |
Peracetic acid |
|
|
Scientific Formula |
HOCl |
C2H4O3 |
|
Production |
Electrolysis of water and salt |
Peracetic acid is prepared from a reaction of acetic acid and hydrogen peroxide, with sulfuric acid as a catalyst. |
|
Color |
Transparent |
Transparent |
|
Efficacy |
Effective against:
|
Effective against:
|
|
Side-effects |
|
|
|
Warning Symbols |
N/A |
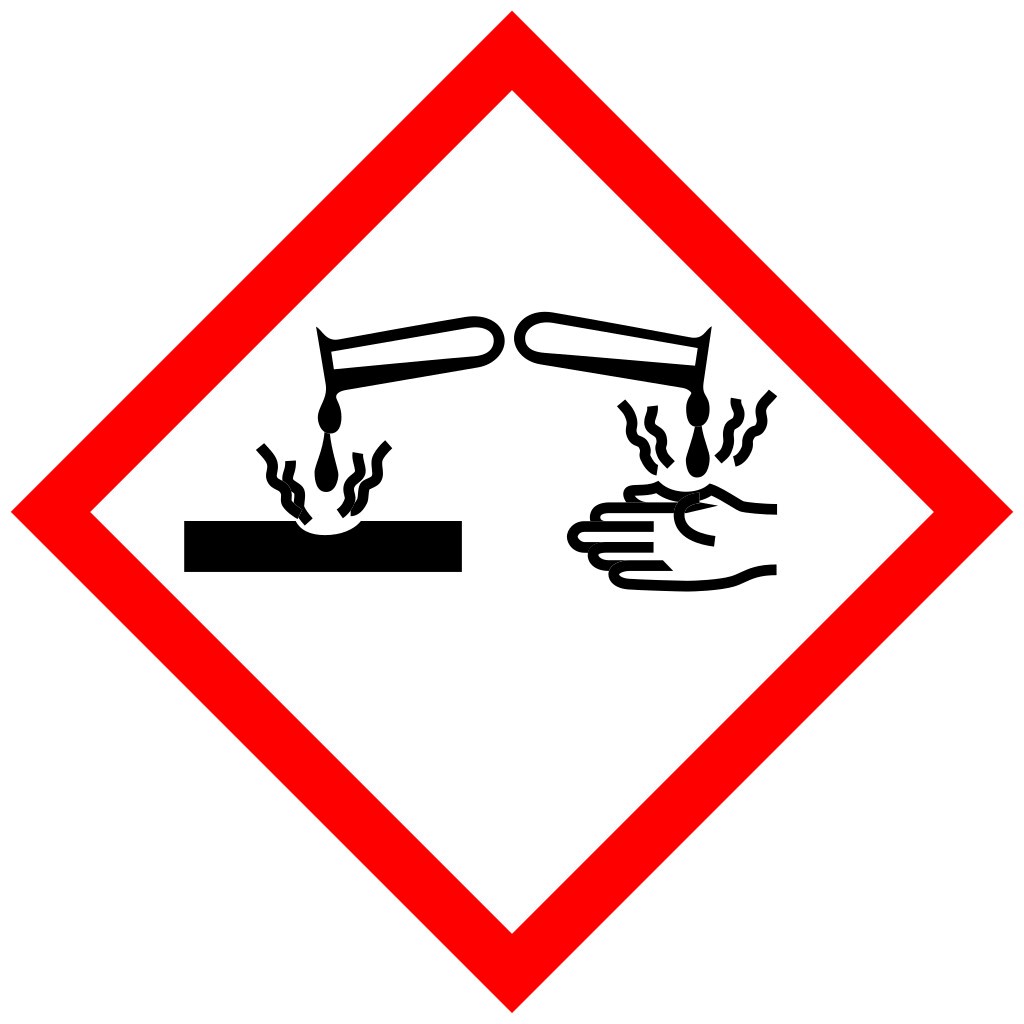  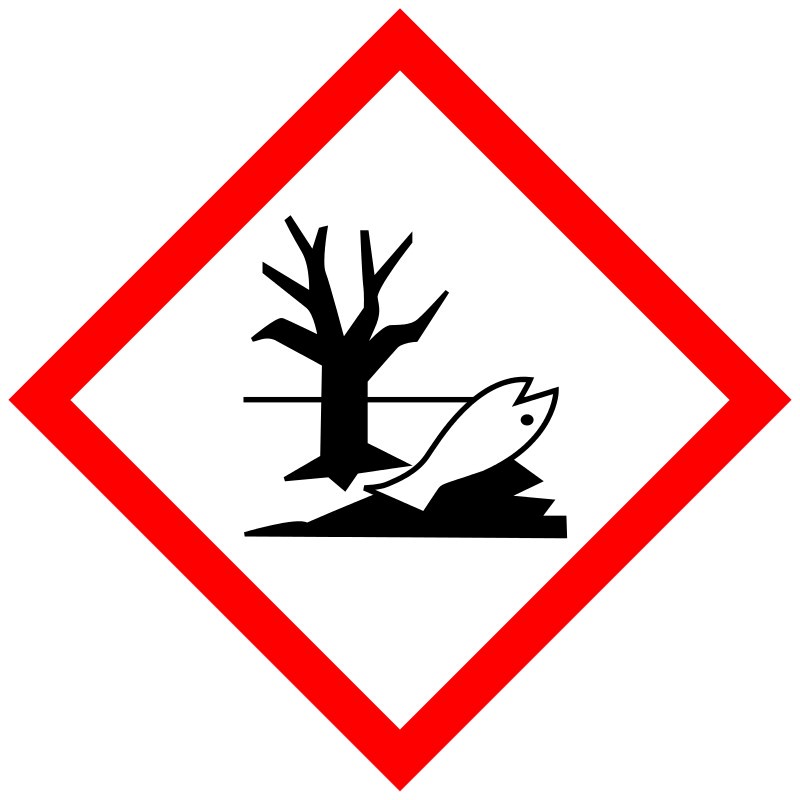  |
Convinced?
That Watter Disinfection Solution is also the perfect alternative to peracetic acid for you?
References
- National Center for Biotechnology Information (2025). PubChem Compound Summary for CID 6585, Peracetic Acid. Retrieved February 12, 2025 from https://pubchem.ncbi.nlm.nih.gov/compound/Peracetic-Acid.
- Tsai, SY., Liu, YM., Lin, ZW. et al. Antimicrobial activity effects of electrolytically generated hypochlorous acid-treated pathogenic microorganisms by isothermal kinetic simulation. J Therm Anal Calorim 148, 1613–1627 (2023). https://doi.org/10.1007/s10973-022-11727-4
- Tsai, SY., Liu, YM., Lin, ZW. et al. Antimicrobial activity effects of electrolytically generated hypochlorous acid-treated pathogenic microorganisms by isothermal kinetic simulation. J Therm Anal Calorim 148, 1613–1627 (2023). https://doi.org/10.1007/s10973-022-11727-4



