Disinfection of process water - The fine balance between corrossion and effectivity
Disinfection of process water
On space exploration missions water is intensively looked for as the indication of extraterrestrial life. This is no coincidence: as far as we know, water is a basic requirement for organic activity. In recent years, water has been frequently found outside our planet, but so far there is no hard evidence for the existence of (micro-)organisms in the cosmos. Personally, I think it is only a matter of time before we will find them, because every QA manager will agree with my statement: where there is water, there are bacteria.

MIC occurs when micro-organisms form biofilms on surfaces and then secrete substances that have a corrosive effect. Biofilm is a persistent, slimy layer of microorganisms. Through biofilm formation, microorganisms can adapt to the environment and thus survive together when they cannot do so alone. For example, bacteria in a biofilm are more resistant to antibiotics and disinfectants than in a loose (planktonic) state.
The development of biofilm
In water pipes biofilm develops as a result of loose (planktonic) microorganisms attaching themselves to the pipe wall. With the help of special molecules called extracellular polymeric substances (EPS), the loose cells make contact with each other and various layers are formed in the biofilm. This enables the biofilm to offer more resistance to the flow of water, for example. Through mutual contact, microorganisms develop differently from each other. This creates a complex structure in which cells start to specialize and thus organisms of the same species perform different tasks. It is a self-contained ecosystem, including specialties such as food acquisition and defense. The outer layer of a biofilm is mainly used for expansion: microorganisms are released and carried away by the water again as planktonic cells. Thus the biofilm colonizes in new places in the pipe.
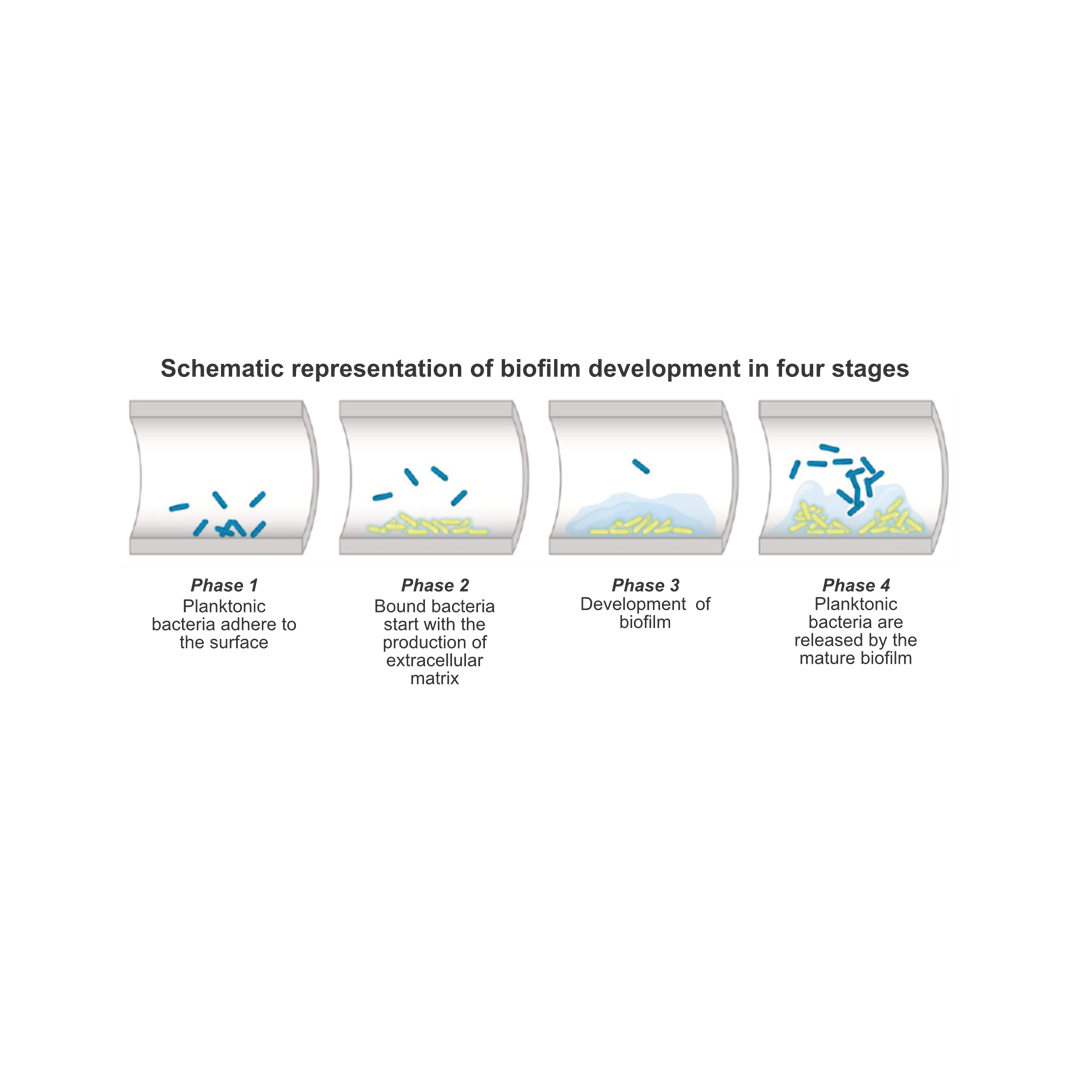
The effective removal and prevention of biofilm is thus crucial for a structural improvement in the quality of process water and associated materials.
Removing biofilm
The cooperation and specialization of a biofilm makes it extra resistant to, among other things, antibiotics, temperature, pressure and acidic or basic environments. This makes a biofilm incredibly difficult to combat properly. It is not only important that the biofilm is broken down, but also that the bacteria are completely killed off continuously, so that they cannot reattach to the pipes to form a new biofilm. However, the proper use of disinfectants is no easy task: not only are there risks related to occupational safety, but the commonly used chemicals can also negatively affect the quality of the water and the final product by creating chemical residues. The dosage of an effective agent is therefore very precise: underdosage creates false safety and resistance among microorganisms, while overdosage causes damage to materials due to the corrosive properties of disinfectants themselves.
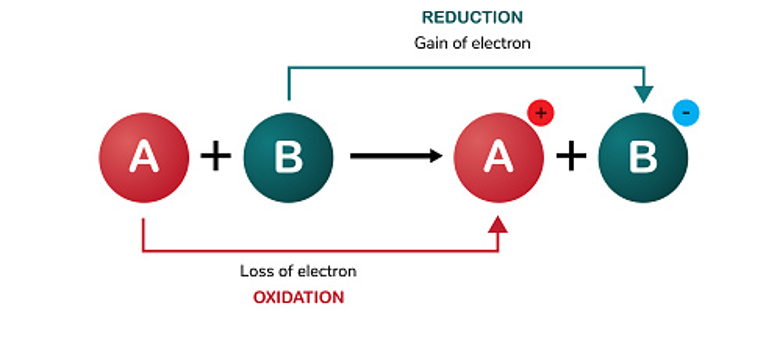
The oxidation-reduction potential
For a correct application of disinfection, it is inevitable to bring in chemistry. To understand how biofilm can be removed (and prevented) without the remedy being worse than the disease, we need to zoom in on the properties of disinfectants. One of the most important concepts in this respect is the reduction-oxidation reaction, abbreviated to redox and indicated by the magnitude ORP (in mV). Briefly, this indicates the power of an agent to remove an oxygen atom and/or to gain electrons (reduction) or to add oxygen atom and/or to lose electrons (oxidation).
Corrosion
Why is this important? Corrosion is the result of redox reactions, the simple example being copper that oxidizes when exposed to oxygen for too long. Corrosion causes materials to change their composition, which in turn causes them to lose their resilience and crumble. As far as corrosion is concerned, a disinfectant with the lowest possible ORP would be preferable.
Disinfection
There are several methods to eliminate microorganisms, but redox reactions play an important role, especially when disinfectants are used. This is because what do common agents such as hydrogen peroxide (H2O2), chlorine dioxide (ClO2), sodium hypochlorite (NaClO; also known as bleach) and peracetic acid (C2H4O3) have in common? A reactive oxygen component. The effectiveness of these agents is therefore based on oxidizing the outer layer of a microorganism. In other words, the higher the redox potential of a disinfectant, the more powerful the disinfectant. In terms of disinfection, a disinfectant with the highest possible ORP would therefore be preferred.
See here: a dilemma.
Effective disinfection
The solution to this seemingly impossible dilemma lies in the dosage required to remove and prevent biofilm. If only very little of an effective disinfectant is required, corrosion will occur more slowly. MIC is thus completely prevented and the damage from chemical corrosion is minimized. Unfortunately, it is impossible to completely eliminate corrosion, because small bits of corrosion will still have a significant effect over time.
Diversity of nature
To find the ideal disinfectant, we need to look beyond the ORP. Microorganisms are extremely diverse and therefore differ in their sensitivity to certain agents. For example, chlorine is effective against bacteria, but fungi are extremely resistant: see also the Wageningen University of Research study on fungi in Dutch swimming pools: link.
The basis for this lies in the anatomy of the various types of microorganisms. Microorganisms are opened up by a cell membrane and a cell wall. The cell membrane is a layer of fat that ensures that the contents of the cell remains inside and the cell wall has the function of structure and protection. In almost all living creatures on earth - from humans to bacteria to banana plants - the structure of the cell membrane is the same: a double layer of phospholipids. The cell wall, on the other hand, can differ enormously from one organism to another.

A city wall
Since the cell wall is the outermost layer of many microorganisms, it is commonly compared to a city wall. It contains gates that can provide access, but overall it is a barrier that keeps unwanted things out. And just like a city wall, the material with which the cell wall is constructed determines the degree of protection against certain attacks. A city wall made of wood is very sensitive to fire, but against one made of stone you have to use quite a few torches to knock it down. It works the same way with microorganisms: the composition of the cell wall makes a fungus more resistant to chlorine than bacteria. And even within the domains of fungus and bacteria, there are many differences from species to species. Since biofilm is a diverse ecosystem of several types of microorganisms, that makes it more resistant to disinfectants than planktonic cells an sich. Not only is there a city wall made of wood, but also of stone, barbed wire and sandbags; the whole is more than the sum of its parts.

Effectiveness
To break through all these barriers, there are actually two solutions. Higher doses of agent can be used (after all, a lot of fire gets stone small too) or multiple active ingredients can be used to combat all types of microorganisms. As explained, higher doses of agents are not preferred because of the increased chemical corrosion that results. For process water, the ideal disinfectant is one with several active ingredients. The problem is, where do you find them?
What about sterilization?
Although sterilization methods, such as UV light or heating, are excellent methods for killing microorganisms without chemical residues, they are unsuitable for disinfection of water pipes. This is because these methods are only locally effective and thus do not prevent contaminants from growing into biofilm in the pipes themselves. For effective disinfection of process water it is important that a disinfectant is effective in the entire system and not just at one point in the system.
In-situ disinfection
Bad news: Mixing chemicals is life-threatening. Good news: producing a broad-spectrum disinfectant can still be done in-house. The future of disinfection is in situ. Apart from the dangerous by-products (read: deadly gases) that mixing different disinfectants produces, stability will also plummet. When several molecules with a high ORP come into contact with each other, they will mainly react with each other. In the time that a jerry can is filled and arrives on location, a disinfectant will have largely lost its effectiveness.
DIY disinfection
However, this can be easily circumvented by producing and using a disinfectant on site. This may seem futuristic, but in the past ten years in situ disinfection technology has made enormous strides. This technology converts water and salt into a broad-spectrum disinfectant, called electrolysed oxidizing water (EOW), by means of an on-site device (Latin: in situ), which can be fed directly into a company's water system.

The in-situ technology produces a disinfectant consisting of more than 20 active ingredients, the main one being HOCl, also known by the names hypochlorous acid or hypochlorous acid. Despite the suffix acid, it is a very friendly agent. This molecule is also produced, for example, by the immune cells of mammals to eliminate microorganisms. Supplemented by various other active substances (including oxygen radicals), the efficacy of disinfectants produced in situ is extremely high. As a result, biofilm can be continuously combated and prevented with very low concentrations of disinfectant, approximately 3-6 ppm. All this culminates in effective disinfection, without the dreaded corrosion.
Win-win-win situation
Because of these properties, in situ technology is making huge strides, particularly in water disinfection. In addition to the aforementioned advantages with regard to effectiveness and lack of corrosiveness, it is also a very attractive option financially. The operational costs are enormously low; tap water and salt are very easily available. In-house production eliminates the need for a supplier, as well as indirect costs of this such as transport, storage, inventory management and processing of plastic and/or chemical waste. Finally, the technology has been used in the Netherlands for many years in the agricultural and food processing industries, so it is out of the development phase. Furthermore, the composition of a disinfectant produced in situ leads to it breaking down relatively easily in water. As a result, there are no chemical residues that can end up in the process water and the disinfectant can be handled without personal protective equipment. Together this makes it a win-win-win situation in terms of effectiveness, profit and safety.
The future of disinfection
For effective, safe disinfection to combat the harmful effects of microorganisms in process water, such as MIC and health risks, every production technologist and QA manager should consider the potential of in-situ technology at their facility. The simple requirements in combination with the effectiveness and safety of the product makes disinfection possible in places where it was not possible before. It is therefore not inconceivable that in situ devices will one day travel to the cosmos to combat undesirable microorganisms. By the way, did you know that the in-situ technology was originally developed by the Soviet space industry?
Curious about our system?
Want to know if the Watter-system is something that might benefit your company? Feel free to ask us, we would love to tell you more.



